

A
color picture tube having a shadow mask provided with a plurality of
vertical aperture rows arranged as horizontally juxtaposed to one
another, each vertical row comprising the electron beam transmissive
apertures vertically arranged in line with a predetermined pitch Py.
With a view to making possibly occurring moires imperceptible, the
arrangement of the apertures are made such that, when spatial deviation
in the vertical positions between any two apertures in the horizontally
adjacent aperture rows is represented by Δy, there may be included
combinations of at least two different type aperture rows of different
deviations Δy which satisfy the following condition ##EQU1## at least
when n is equal to 1, 2, 3 or 4 and k is an odd number smaller than 2n.
The
present invention relates in general to a color picture tube with a
shadow mask, and in particular to a color picture tube having a shadow
mask which is provided with a plurality of electron beam transmissive
aperture rows extending perpendicularly to the scanning lines and each
comprising a plurality of the individual rectangular apertures for
transmission of the electron beams arrayed in line with a predetermined
pitch.
Lately, with an effort to simplify the structure
of deflection system and at the same time to enhance the visual
sharpness of the produced image of the color picture tube with the
shadow mask such as color cathode ray tube, Braun tube or the like
(hereinafter referred to also as CPT), there have been developed and
increasingly employed CPT's of the shadow mask type in which the shadow
mask is provided with aperture rows extending orthogonally to the
scanning lines and each comprising a plurality of the electron beam
transmissive rectangular apertures (hereinafter also referred to as
apertures) arrayed vertically in line with a predetermined vertical
pitch and in which three electron guns are arrayed in line, in place of
the heretofore known color picture with shadow mask in which circular
phosphor dots are arrayed in a form of equilateral triangle. However,
the color picture tube of the above type suffers from the drawbacks that
a strip or fringe pattern, that is, moire of a great pitch is produced
as a result of the interaction between shades of the bridge portions
between the rectangular apertures formed vertically in a repeated
pattern with the predetermined pitch and the bright-dark portions of the
scanning lines, thereby to deteriorate the picture quality of the
produced image.
Many and various attempts have been
hitherto proposed for reducing the moire phenomenon. According to a
known method, the apertures of the horizontally adjacent aperture rows
are deviated from one another in respect of the vertical position for a
distance of 1/αPy where α is an integer and Py is the vertical pitch of
the aperture row. This method starts from two observations. Namely, on
one hand, the moire is determined by the scanning lines and the
deviation, since the moire pitch becomes greater as the difference
between the pitch of the scanning line and the vertical pitch of the
apertures in the rows is selected smaller and since the deviation causes
horizontal fringe whose pitch is Py /α. In other words, the deviation
in the vertical position between the horizontally adjacent rows will
bring about a shade patern in the substantially horizontal direction and
the moire will become more imperceptible as the magnitude of the
deviation is selected smaller because the ratio between the pitch of the
scanning line and the pitch of the shade pattern will then become
large. On the other hand, in accordance with the other observation, the
horizontal pattern of shade, i.e. interlaced dark and bright portions
will not be produced if the integrated value of the electron
transmissivity or through rate of the apertures remains the same for
each of the sanning lines. Accordingly, the moire can be suppressed by
adjusting the deviation and the width of the bridge portions between the
vertically aligned aperture in a row. However, the inventors have found
after repeated experiments that the hitherto proposed method as
described above can not make the moire in oblique directions
imperceptible although the method is certainly effective in suppressing
the moire appearing in a form of bright and dark pattern in the vertical
position.
It has also been proposed to array the
apertures in a random pattern. However, this solution meets with
difficulties in the manufacturing thereof.
Accordingly,
a main object of the invention is to provide a color picture tube of
the shadow mask type in which the moire is made imperceptible.
Another
object of the invention is to realize a shadow mask for a color picture
tube which is effective in suppressing the occurrence of the moire.
Still
another object of the invention is to reduce undesirable influences of
moire due to the harmonics of the luminance distribution pattern of the
scanning lines and of the transmissivity or through rate pattern of the
vertically arrayed apertures and the poor linearity of the vertical
distribution pattern of the scanning lines.
A further
object of the invention is to provide a color picture tube which can be
employed commonly in NTSC (National Television System Commitee), PAL
(Phase Alternation By Line) and SECAM (Sequential a Memoire) color
television systems without any appreciable moire.
With
the above objects in view, the present invention contemplates to
determine the array of the apertures so that the pitch and phase of beat
components, ie moires, produced by the mutual product of the vertical
transmissivity or through rate distribution pattern of the aperture row
and the vertical luminance distribution pattern of the scanning lines
will take predetermined values.
More specifically, the
shadow mask according to the present invention is so constructed as to
comprise at least two different types of the aperture rows of different
deviations which fulfill the following condition: ##EQU2## wherein Py
represents the pitch of apertures in the vertical aperture row, Δy
represents the vertical deviation between the apertures in the
horizontally adjacent aperture rows, n is a positive interger of 1 to 5
and k is an odd number smaller than 2n.
The shadow mask
according to the invention provides significant advantages particularly
when the luminance distribution pattern of the scanning line cannot be
approximated by a sine wave or when the face plate of the color picture
tube is remarkably curved at peripheral portions.
MITSUBISHI cathode ray tube:
A cathode ray tube
comprises a face plate glass containing neodymium oxide (Nd2 O3); and a
phosphor screen of plural color phosphors formed on an inner surface of
said face plate glass wherein a phosphor of zinc sulfide activated with
copper and aluminum (ZnS:Cu, Al) or a phosphor of zinc sulfide
activated with copper, gold and aluminum (ZnS:Cu, Au, Al) is used as a
green phosphor of said phosphor screen.
Inventors:
Iwasaki, Yasuo (Kyoto, JP)
Yajima, Koji (Kyoto, JP)
Mitsubishi Denki Kabushiki Kaisha (Tokyo, JP)
This application is a continuation of application Ser. No. 338,993, filed Jan. 12, 1982 now abandoned.
1. Field of the Invention
The present invention relates to a color cathode ray tube having a phosphor screen.
2. Description of the Prior Art
It
has been proposed to reduce a transmittance of a face plate glass on a
phosphor screen as an effective manner for improving image contrast of a
phosphor screen of a cathode ray tube.
Referring to FIG. 1, the principle will be illustrated in detail.
FIG. 1 is a sectional model of the phosphor screen of the color cathode ray tube wherein the reference numeral (1) designates a face plate glass on an inner surface of which three color phosphors (2) of red (R), green (G) and blue (B) are formed.
The following equations are give: E1 =Eo Rp Tf2 (I) F1 =Fo Tf (II)
wherein Eo represents an intensity of exterior incident light to the face plate glass (1) of the color cathode ray tube having the structure; E1 represents an intensity of reflective light reflected on the phosphor screen to emit out of the face plate glass (1); Tf represents a light transmittance of the face plate glass (1); Rp represents reflectance of the red (R) green (G) blue (B) three color phosphors (2); Fo represents an intensity of light emission of three color phosphors and F1 represents an output of light emitted from the phosphor screen and passed through the face plate glass (1).
A contrast C is defined by the equation: ##EQU1## Thus, the following equation is given by substituting (I) and (II) in (III): ##EQU2##
In precise calculation, it is necessary to apply factors caused by effects of reflection of exterior light on the surface of the face plate glass, multiple reflections in the face plate glass (1) and halation caused by scattered electrons. Thus, the effects are neglected because the effects are negligible.
In order to improve the contrast of images of the cathode ray tube, the light transmittance (Tf) of the face plate glass (1) must be reduced as clearly considered by the equation (IV).
The glass used as the face plate glass (1) of the cathode ray tube has been classified into a clear glass having a transmittance of 75% or more; a grey glass having a transmittance of 60-75% and a tint glass having a transmittance of 60% or less.
FIG. 2 show typical spectral transmittance curves of (a) a clear glass, (b) a grey glass and (c) a tint glass and also
emission spectra of the three color phosphors of red (R), green (G), blue (B).
On the other hand, as it is clearly found from FIG. 2 and the equation (II), the output of light emitted from the phosphor screen as brightness of the phosphor screen decreases depending upon a decrease of the transmittance (Tf) of the face plate glass (1). This is opposite to the contrast. In view of the transmittance (Tf) of the face plate glass (1), both of the contrast characteristic of images and the brightness characteristic are not easily improved. The kind of the face plate glass (1) has been selected depending upon the weight of the contrast or the brightness characterstic.
It has been defined to give selective photo-absorption for the face plate glass (1) in the region of small light emission energy as the wavelength region in roots of the emission spectra of the three color phosphors on the phosphor screen instead of the face plate glass having flat transmittance in visible wavelength region as shown in FIG. 2 in order to overcome the difficulty for improving both the brightness and the contrast and to improve both the brightness characteristic and the contrast characteristic.
FIG. 3 shows a spectral transmittance curve of the face plate glass (1) proposed for the aforementioned purposes.
The face plate glass is formed by incorporating neodymium oxide (Nd2 O3) (at 1.0 wt. %) in a glass formulation similar to those of the conventional clear glass (hereinafter referring to as Nd-containing glass). The Nd-containing glass has main sharp absorption peak in 560-615 nm and sub-absorption peaks in 490-540 nm, which is resulted by the specific characteristics of Nd2 O3. The absorption peaks are quite sharp and accordingly, even though light transmittances in the other wavelength except the absorption peaks are remarkably high as those of the conventional clear glass an average light transmittance in the all visible wavelength region is similar to those of the grey glass thereby contributing to the improvement of the contrast.
FIG. 4 shows spectral transmittance curve (d) of the Nd-containing glass and emission spectra of the three color phosphors of red (R), green (G), blue (B) of the color cathode ray tube.
When the Nd-containing glass is used for the face plate glass, the brightness characteristic of the phosphor screen and the contrast characteristic are remarkably improved. However, the body color of the phosphor screen is quite different from those of the conventional color cathode ray tubes to cause uneasy feeling for a spectator in appearance.
Referring to FIG. 5, the body color of the phosphor screen will be illustrated in detail. In FIG. 5, the points A, B, C are typical
chromaticity points of white exterior light plotted on a CIE chromaticity diagram in the case of watching a TV set at home. The A point is the chromaticity point of the A standard light source which is similar to the chromaticity point of light of an incandescent lamp used at home. The B point is one example of chromaticity points of light of white flurescent lamp used at home. The C point is a chromaticity point of the C standard light source as an average daylight.
When the spectral reflectance of the phosphors (2) of the phosphor screen is substantially flat in the visible wavelength region and the spectral transmittance of the face plate glass (1) is substnatially flat in the visible wavelength region as that of the clear glass, the chromaticity point of the reflected exterior light reflected from the phosphor screen, that is the body color of the phosphor screen is similar to the chromaticity point of the exterior lighter.
On the other hand, when the Nd-containing glass is used for the face plate glass on the phosphor screen, the spectral transmittance of the face plate glass is not flat in the visible wavelength region but has complicated cuves. Thus, the chromaticity point of the reflected exterior light reflected from the phosphor screen, that is, the body color of the phosphor screen is different from the chromaticity point of the white exterior light.
The case of the illuminant A (A point shown in FIG. 5) will be illustrated. In the case of the exterior light from the illuminant A, the exterior light incident to the phosphor screen is reflected by the phosphors (2) in substantially flat form in the visible wavelength, however, the component of wavelength of the reflected exterior light is different from that of the incident exterior light because of the sharp absorption at 580 nm and sub-absorption in the sub-absorption band at 530 nm of the Nd-containing glass. The effect is shown in the CIE chromaticity diagram to find the following fact. The main absorption band at 580 nm results in a reduction of the component of the wavelength at the band of the exterior light whereby the chromaticity point is affected to depart from the single color chromaticity point (Q) at 580 nm on the line (β) connecting the single color chromaticity point (Q) at 580 nm and the chromaticity point (A) of the A light source. (This is shown by the vector a2.)
The sub-absorption band at 530 nm results in a reduction of the component of the wavelength at the band of the exterior light whereby the chromaticity point of the reflected exterior light is affected to depart from the single color chromaticity point (R) at 530 nm on the line (α) connecting the single color chromaticity point (R) at 530 nm and the chromaticity point (A) of the illuminant A. (This is shown by the vector a1.) Therefore, the chromaticity point of the reflected exterior light, that is, the body color of the phosphor screen is shifted to the vector a3 as a combination of the vectors a1 and a2. The absorption in the main absorption band is remarkably greater than that of the subabsorption band. Thus, the absolute value of the vector a2 is remarkably greater than the absolute value of the vector a1.

In the cases of the white fluorescent lamp (B point) and the illuminant C (C point), the chromaticity point of the reflected exterior light, that is, the body color of the phosphor screen is respectively shifted in the direction of the vector b3 or the vector c3. In these cases, the absolute value of the vector b2 or the vector c2 is respectively greater than that of the vector b1 or the vector c1 because of the great difference of the absorptions in the main absorption band and the sub-absorption band. As described, when the Nd-containing glass is used as a face plate glass, the body color of the phosphor screen is different from the chromaticity of the white exterior light to be unstable. This is not preferable in view of the apperance of the phosphor screen.
SUMMARY OF THE INVENTION
It is an
object of the present invention to overcome a disadvantage of unstable
body color of a phosphor screen which causes by using a Nd-containing
glass as a face plate glass of a color cathode ray tube.
It is
another object of the present invention to provide a color cathode ray
tube having stable body color of a phosphor screen in the use of a face
plate glass made of a Nd-containing glass.
The foregoing and
other objects of the present invention have been attained by providing a
cathode ray tube which comprises a face plate glass containing
neodymium oxide (Nd2 O3); and a phosphor screen of plural color
phosphors formed on an inner surface of said face plate glass wherein a
phosphor of zinc sulfide activated with copper and aluminum (ZnS:Cu, Al)
is used as a green phosphor of said phospor screen.
Claims:
We claim:
1.
A cathode ray tube having a face plate glass which contains neodymium
oxide (Nd2 O3) and exhibits a sharp main absorption band having a peak
at about 580 nm and a sub-absorption at about 530 nm and on the inner
surface of which a phosphor screen having blue, green and red phosphors
is formed, wherein said blue phosphor is a phosphor of zinc sulfide
activated with Ag(ZnS:Ag), said red phosphor is a phosphor of yttrium
oxysulfide activated with Eu(Y2 O2 S:Eu), and said green phosphor is a
phosphor of zinc sulfide activated with copper and aluminum (ZnS:Cu, Al)
to reduce the spectral reflectance of said phosphor screen at the
wavelength band of 470-480 nm so that the chromaticity point of a
reflected exterior light reflected from said phosphor screen is
prevented from displacement with respect to the chromaticity point of an
exterior light due to the influence of said main and sub-absorption
bands.
2. The cathode ray tube according to claim 1, wherein a
content of copper (Cu) as an activator is 3×10-4 g, or more to 1 g. of
zinc-sulfide (ZnS) as the main component.
3. The cathode ray tube according to claim 1, wherein each of said blue and red phosphors is a phosphor with a colorant.
4.
A cathode ray tube having a face plate glass which contains neodymium
oxide (Nd2 O3) and exhibits a sharp main absorption band having a peak
at about 580 nm and a sub-absorption band at about 530 nm and on the
inner surface of which a phosphor screen having blue, green and red
phosphors is formed, wherein said blue phosphor is a phosphor of zinc
sulfide activated with Ag(ZnS:Ag), said red phosphor is a phosphor of
yttrium oxysulfide activated with Eu(Y2 O2 S:Eu) and said green phosphor
is a phosphor of zinc sulfide activated with copper, gold and aluminum
(ZnS:Cu, Au, Al) to reduce the spectral reflectance of said phosphor
screen at the wavelength band of 470-480 nm so that the chromaticity
point of a reflected exterior light reflected from said phosphor screen
is prevented from displacement with respect to the chromaticity point of
an exterior light due to the influence of said main and sub-absorption
bands.
5. The cathode ray tube according to claim 4, wherein a
content of gold (Au) as an activator is 4×10×4 g. or more to 1 g. of
zinc-sulfide (ZnS) as the main component.
6. The cathode ray tube according to claim 4, wherein each of said blue and red phosphors is a phosphor with a colorant.
Description:
BRIEF DESCRIPTION OF THE DRAWINGS
FIG. 1 is a sectional model of a phosphor screen of a cathode ray tube;
FIG. 2 shows typical spectral transmittance curves of various glasses;
FIG. 3 shows a spectral transmittance curve of the Nd-containing glass;
FIG. 4 shows spectral transmittance curves of the Nd-containing glass and a clear glass;
FIG. 5 is a CIE chromaticity diagram plotting chromaticity points of white exterior light on a white diagram;
FIGS.
6 to 8 respectively show spectral reflection characteristics of three
color phosphors made of various combinations of phosphors;
FIG. 9 is a CIE chromaticity diagram plotting chromaticity points of body colors of various phosphor screens;
FIGS.
10 to 12 respectively show spectral reflection characteristics of three
color phosphors made of various combinations of phosphors;
FIG. 13 is a CIE chromaticity diagram plotting chromaticity points of body colors of various phosphor screens;
FIGS.
14 and 15 respectively show spectral reflection characteristics of
three color phosphors made of various combinations of phosphors;
FIG. 16 is a CIE chromaticity diagram plotting chromaticity points of body colors of various phosphor screens;
FIGS.
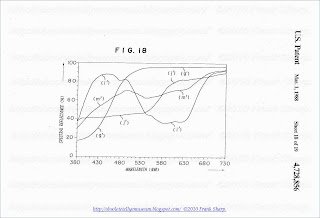
17 and 18 respectively show spectral reflection characteristics of
three colors phosphors made of various combinations of phosphors;
FIG. 19 is a CIE chromaticity diagram plotting chromaticity points of body colors of various phosphor screens.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
Referring
to FIGS. 5 to 13, one embodiment of the present invention will be
illustrated. Firstly, referring to FIG. 5, the principle of the present
invention will be illustrated.
When the Nd-containing glass is
used for the face plate glass on the phosphor screen, the body color of
the phosphor screen is highly changed by the vector (a2, b2, c2) on the
chromaticity diagrams which is caused by the main absorption band at 580
nm and the vector (a1, b1, c1) which is caused by the sub-absorption
band at 530 nm. Thus, the vector (a1, b1, c1) is remarkably less than
the vector (a2, b2, c2). In the present invention, the stability of the
body color of the phosphor screen is considered in view of the vector
(a2, b2, c2). In order to eliminate the effect of the vector (a2, b2,
c2), it is preferable to result in a reverse vector (-a2, -b2, -c2).
The
method of formation of the reverse vector in the case of the
chromaticity point of the illuminant A will be illustrated. The cross
point (Qa point) of the line β connecting the single color chromaticity
point Q of the main absorption band at 580 nm and the chromaticity (A)
of the illuminant A to the contour line is a single color chromaticity
point at about 470 nm. When the light component at about 470 nm among
the light components of the reflected exterior light is reduced at a
desired degree by a suitable manner, the chromaticity point is shifted
to depart from the single color chromaticity point Qa on the line β
connecting the single color chromaticity point Qa at about 470 nm and
the chromaticity point (A) of the illuminant A whereby the vector -a2 is
resulted. In the cases of the other kinds of the white exterior lights,
the cross point Qb, Qc of the line εδ and the contour line are at about
480 nm. The light component at about 480 nm among the light components
of the reflected exterior light is reduced at a desired degree whereby
the vectors -b2 and -c2 are formed.
When the Nd-containing glass
is used for the face plate glass on the phosphor screen, the change of
the body color on the phosphor screen can be substantially eliminated by
reducing the light components at 470-480 nm of the reflected exterior
light.
In order to reduce the light components at 470-480 nm
among the light components of the reflected exterior light, it is enough
to reduce the spectral reflectance of the three color phosphors (2) at
the wavelength band of 470-480 nm.
The inventors have studied
stability of the body color of the phosphor screen in the combinations
of various phosphors having desired emission spectrum for the three
color phosphors of the phosphor screen of the color cathode ray tube and
the face plate glass made of the Nd-containing glass. As a result, it
has been to provide a phosphor screen having stable body color by using a
phosphor zinc sulfide activated with copper and aluminum (ZnS:Cu, Al)
as the green (G) phosphor among the three color phosphors (2).
Referring to FIGS. 6 to 13, the embodiment of the invention will be further illustrated.
FIG. 6 shows spectral reflection characteristics given by an undesired combination of phosphors for the three color
phosphors (2). More particularly, FIG. 6 shows spectral reflection characteristics of a phosphor of gadolinium oxysulfide activated with Tb (Gd2 O2 S:Tb) as a green (G) phosphor; a phosphor of zinc sulfide activated wiht Ag (ZnS:Ag) as a blue (B) phosphor and a phosphor of yttrium oxysulfide activated with Eu (Y2 O2 S:Eu) as the three color phosphors (2) and a spectral reflection characteristic of the three color phosphors (2) as a combination of said phosphors. In FIG. 6, the reference (a), (b), (c) and (d) respectively represent spectral reflectance curves of the Gd2 O2 S:Tb phosphor, the ZnS:Ag phosphor, the Y2 O2 S:Eu phosphor and the three color phosphors (2) thereof.
The spectral reflectance curve (d) of the three color phosphors (2) is not substantially reduced at 470-480 nm and accordingly, it is not desired in view of the body color of the phosphor screen.
FIG. 7 shows spectral reflection characteristic of a desired combination of the three color phosphors (2).
The blue (B) phosphor and the red (R) phosphor are the same as the phosphors in FIG. 6 as the ZnS:Ag phosphor and the Y2 O2 S:Eu phosphor. Thus, the green (G) phosphor is the ZnS:Cu, Al phosphor which has a spectral reflectance curve (e). Thus, the three color phosphor (2) has the spectral reflectance curve (f) which has the reduction at 470-480 nm. Therefore, the desired body color of the phosphor screen is given by the combination of the three color phosphors and the face plate glass made of a Nd-containing glass. This is resulted by great reduction of the spectral reflectance curve (e) of the ZnS:Cu, Al phosphor used as the green (G) phosphor of the three color phosphors (2) in the shorter wavelength side of the visible wavelength region. The reduction of the spectral reflectance curve in the shorter wavelength side of the visible wavelength region is highly affected by a content of Cu as the activator for the phosphor. In the case of the ZnS:Cu, Al phosphor having the spectral reflectance curve (e), 1 g. of zinc sulfide (ZnS) is activated with about 1×10- 4 g. of Cu as the activator. The reduction of the spectral reflectance curve in the shorter wavelength side of the visible wavelength region increases depending upon an increase of the content of Cu as the activator.
FIG. 8 shows the spectral reflectance curves in the case of the increase of the content of Cu as the activator in the ZnS:Cu,
Al phosphor used as the green (G) phosphor. The blue (B) phosphor and the red (R) phosphor are respectively the ZnS:Ag phosphor and the Y2 O2 S:Eu phosphor shown in FIGS. 6 and 7. In this case, 1 g. of zinc sulfide is activated with about 5×10-4 g. of Cu as the activator. The spectral reflectance curve of the ZnS:Cu, Al phosphor is the curve (g) in which the reduction in the shorter wavelength side of the visible wavelength region is greater than that of the curve (e) in FIG. 7. As result, the spectral reflectance curve of the three color phosphors (2) is the curve (h) in which the reduction in the shorter wavelength of the visible wavelength region is greater than that of the curve (f) in FIG. 7.
FIG. 9 is a CIE chromaticity diagram plotting the body color of the phosphor screen by the light of the illuminant A as the white exterior light in the case of the formation of the three color phosphors (2) in said combination on the face plate glass made of a Nd-containing glass. In FIG. 9, the reference (A) designates a chromaticity point of the A light source; and the reference (P) designates a chromaticity point of the reflected exterior light from the phosphor screen, as the body color of the phosphor screen in the case of the formation of the three color phosphors (2) having substantially flat spectral reflectance as shown in FIG. 6(d) as the combination of the Gd2 O2 S:Tb green (G) phosphor, the ZnS:Ag blue (B) phosphor and the Y2 O2 S:Eu red (R) phosphor on an inner surface of the face plate glass made of the clear glass. The position (P) is slightly different from the position (A) because the spectral transmittance of the clear glass is not completely flat but has slightly difference and the spectral reflectance of the three color phosphors (2) has slightly difference. The reference (E) designates a chromaticity point of the reflected exterior light from the phosphor screen, as the body color of the phosphor screen in the case of the formation of the three color
phosphors (2) shown in FIG. 6 as the undesired combination of the Gd2 O2 S:Tb green (G) phosphor, the ZnS:Ag blue (B) phosphor and the Y2 O2 S:Eu red (R) phosphor on an inner surface of the face plate glass made of the Nd-containing glass. In this case, the body color of the phosphor screen is remarkably shifted from the chromaticity point (A) of the illuminant A as the white exterior light whereby unstable chromaticity is given and the appearance of the phosphor screen is not preferable. The reference (F) designates a chromaticity point of the reflected exterior light from the phosphor screen, as the body color of the phosphor screen in the case of the formation of the three color phosphor shown in FIG. 7 as the desired combination of the ZnS:Cu, Al green (G) phosphor, the ZnS:Ag blue (B) phosphor and the Y2 O2 S:Eu red (R) phosphor on an inner surface of the face plate glass made of the Nd-containing glass. As described, the light component at 470-480 nm in the reflected exterior light is reduced and the shift from the chromaticity point (A) of the illuminant A is remarkably smaller than that of the (E) point.
The reference (G) designate the chromaticity point as the body color of the phosphor screen of the three color phosphors (2) containing the ZnS:Cu, Al phosphor having larger content of Cu as the activator. The shift from the chromaticity point (A) of the illuminant A is less than that of the (F) point.
In view of the contribution for the stability of the body color of the phosphor screen used with the face plate glass made of the Nd-containing glass. The content of Cu as the activator in the ZnS:Cu, Al phosphor is preferably 5×10-5 g. or more to 1 g. of zinc sulfide as the main component. The stability of the body color of the phosphor screen is further improved by the content of Cu of 3×10-4 g. or more to 1 g. of zinc sulfide.
FIGS. 10 to 12 show the spectral reflectance curves of the ZnS:Ag blue phosphor with a blue colorant, as a blue (B)
phosphor the Y2 O2 S:Eu phosphor with a red colorant as a red (R) phosphor which are recently used to improve the contrast of the phosphor screen and various green (G) phosphor and the three color phosphors as the combinations (FIGS. 10 to 12) and a CIE chromaticity diagram FIG. 13 plotting body colors of the phosphor screens of said three color phosphors formed on the inner surface of the face plate glass made of the clear glass or the Nd-containing glass by using the light of the illuminant A as the white exterior light (FIG. 13) which are shown as FIGS. 6 to 9.
In FIGS. 10 to 12, the curve (i) is a spectral reflectance curve of the ZnS:Ag phosphor with a blue colorant the curve (j) is a spectral, reflectance curve of the Y2 O2 S:Eu phosphor with a red colorant. A spectral reflectance curve of the three color phosphors (2) as the combination of these blue and red phosphors and the Gd2 O2 S:Tb phosphor is the curve (k) in which the reduction of the light component at 470-480 nm is not substantially found. When the ZnS:Cu, Al phosphor is combined with these blue and red phosphors, the spectral reflectance curve is the curve (1) shown in FIG. 11 in which the light component at 470-480 nm is remarkably reduced.
When the ZnS:Cu, Al phosphor having large content of Cu as the activator is combined with these blue and red phosphors, the spectral reflectance curve is the curve (m) shown in FIG. 12 in which the light component at 470-480 nm is further reduced.
FIG. 13 is a CIE chromaticity diagram plotting body colors of the phosphor screens of the three color phosphors as the
combinations thereof which are respectively formed on the face plate glass made of the clear glass or the Nd-containing glass by the light of the illuminant A as the white exterior light. The reference (A) designates the chromaticity point of the illuminant A. The reference (H) designates the chromaticity point of the reflected exterior light from the phosphor screen of the three color phosphors (2) as the combination of the Gd2 O2 S:Tb phosphor, the ZnS:Ag phosphor with a blue colorant and the Y2 O2 S:Eu phosphor with a red colorant formed on the inner surface of the face plate glass (1) made of the clear glass as the chromaticity point of the body color of the phosphor screen. The reference (I) designates the chromaticity point of the reflected exterior light from the phosphor screen of the three color phosphors (2) as the combination of the Gd2 O2 S:Tb phosphor, the ZnS:Ag phosphor with a blue colorant and the Y2 O2 S:Eu phosphor with a red colorant formed on the inner surface of the face plate glass (1) made of the Nd-containing glass as the
chromaticity point of the body color of the phosphor screen. The reference (J) designates the chromaticity point of the
reflected exterior light from the phosphor screen of the three color phosphors (2) as the combination of the ZnS:Cu, Al phosphor, the ZnS:Ag phosphor with a blue colorant and the Y2 O2 S:Eu phosphor with a red colorant formed on the inner surface of the face plate glass (1) made of the Nd-containing glass as the chromaticity point of the body color of the phosphor screen. The reference (K) designates the chromaticity point of the reflected exterior light from the phosphor screen of the three color phosphors (2) as the combination of the ZnS:Cu, Al phosphor having larger content of Cu as the activator, the ZnS:Ag phosphor with a blue colorant and the Y2 O2 S:Eu phosphor with a red colorant formed on the inner surface of the face plate glass (1) made of the Nd-containing glass as the chromaticity point of the body color of the phosphor screen. As it is clearly found from the chromaticity points, the light component at 470-480 nm of the reflected exterior light can be reduced by the combination of the ZnS:Cu, Al phosphor as the green (G) phosphor with the blue colorant-coated blue (B) phosphor and the red colorant-coated red (R) phosphor, whereby the unstable body color caused by the use of the face plate glass (1) made of the Nd-containing glass is remarkably improved. That is, the chromaticity point of the body color is shifted from (I) to (J). When the content of Cu as the activator in the ZnS:Cu, Al phosphor is increased, the stability of the body color of the phosphor screen is further improved by further reducing the light component at 470-480 nm. That is, the chromaticity point is shifted to (K).
The embodiment using the A light source as the white exterior light source has been described. Thus, the same effect is given by reducing the light component at 470-480 nm of the reflected exterior light by the combination of the ZnS:Cu, Al phosphor as the green (G) phosphor in the case of the other white exterior light source such as the white fluorescent lamp and the C light source.
Recently, a black matrix type phosphor screen having light absorption layer between the three color phosphors has been used for improving the contrast of the phosphor screen. The present invention can be applied for such phosphor screen.
In accordance with the present invention, the unstable body color of the phosphor screen caused by the use of the face plate glass made of the Nd-containing glass can be overcome by the combination of the three color phosphors using the ZnS:Cu, Al phosphor as the green (G) phosphor whereby the phosphor screen for the stable body color is given and the contrast and brightness characteristics are improved to provide a cathode ray tube having high quality.
Referring to FIGS. 14 to 19 the other embodiment of the present invention will be illustrated.
In this embodiment, a phosphor of zinc sulfide activated with copper, gold and aluminum (ZnS:Cu, Au, Al) is used as the green (G) phosphor.
FIG. 14 shows spectral reflection characteristic of a desired combination of the three color phosphors (2).
The blue (B) phosphor and the red (R) phosphor are the same as the phosphors in FIG. 6 as the ZnS:Ag phosphor and the Y2 O2 S:Eu phosphor. Thus, the green (G) phosphor is the ZnS:Cu, Au, Al phophor which has a spectral reflectance curve (e'). Thus, the three color phosphor (2) has the spectral reflectance curve (f') which has the reduction at 470-480 nm. Therefore, the desired body color of the phosphor screen is given by the combination of the three color phosphors and the face plate glass made of a Nd-containing glass. This is resulted by great reduction of the spectral reflectance curve (e') of the ZnS:Cu, Au, Al phosphor used as the green (G) phosphor of the three color phosphors (2) in the shorter wavelength side of the visible wavelength region. The reduction of the spectral reflectance curve in the shorter wavelength side of the visible wavelength region is highly affected by a content of Au as the activator for the phosphor. In the case of the ZnS:Cu, Au, Al phosphor having the spectral reflectance curve (e'), 1 g. of zinc sulfide (ZnS) is activated with about 2×10-4 g. of Au as the activator. The reduction of the spectral reflectance curve in the shorter wavelength side of the visible wavelength region increases depending upon an increase of the content of Au as the activator.
FIG. 15 shows the spectral reflectance curves in the case of the increase of the content of Au as the activator in the Zn S:Cu,
Au, Al phosphor used as the green (G) phosphor. The blue (B) phosphor and the red (R) phosphor are respectively the ZnS:Ag phosphor and the Y2 O2 S:Eu phosphor shown in FIGS. 6 and 15. In this case, 1 g. of zinc sulfide is activated with about 1.5×10-3 g. of Au as the activator. The spectral reflectance curve of the ZnS:Cu, Au, Al phosphor is the curve (g') in which the reduction in the shorter wavelength side of the visible wavelength region is greater than that of the curve (e') in FIG. 14. As result, the spectral reflectance curve of the three color phosphors (2) is the curve (h') in which the reduction in the shorter wavelength of the visible wavelength region is greater than that of the curve (f') in FIG. 14.
FIG. 16 is a CIE chromaticity diagram plotting the body color of the phosphor screen by the light of the A light source as the white exterior light in the case of the formation of the three color phosphors (2) in said combination on the face plate glass made of a Nd-containing glass. In FIG. 9, the reference (A) designates a chromaticity point of the A light source; and the reference (P) designates a chromaticity point of the reflected exterior light from the phosphor screen, as the body color of the phosphor screen in the case of the formation of the three color phosphors (2) having substantially flat spectral reflectance as shown in FIG. 6(d) as the combination of the Gd2 O2 S:Tb green (G) phosphor, the ZnS:Ag blue (B) phosphor and the Y2 O2 S:Eu red (R) phosphor on an inner surface of the face plate glass made of the clear glass. The position (P) is slightly different from the position (A) because the spectral
transmittance of the clear glass is not completely flat but has slightly difference and the spectral reflectance of the three color phosphors (2) has slightly difference. The reference (E) designates a chromaticity point of the reflected exterior light from the phosphor screen, as the body color of the phosphor screen in the case of the formation of the three color phosphors (2) shown in FIG. 6 as the undesired combination of the Gd2 O2 S:Tb green (G) phosphor, the ZnS:Ag blue (B) phosphor and the Y2 O2 S:Eu red (R) phosphor on an inner surface of the face plate glass made of the Nd-containing glass. In this case, the body color of the phosphor screen is remarkably shifted from the chromaticity point (A) of the illuminant A as the white exterior light whereby unstable chromaticity is given and the appearance of the phosphor screen is not preferable. The reference (F') designates a chromaticity point of the reflected exterior light from the phosphor screen, as the body color of the phosphor screen in the case of the formation of the three color phosphor shown in FIG. 14 as the desired combination of the ZnS:Cu, Au, Al green (G) phosphor, the ZnS:Ag blue (B) phosphor and the Y2 O2 S:Eu red (R) phosphor on an inner surface of the face plate glass made of the Nd-containing glass. As described, the light component at 470-480 nm in the reflected exterior light is reduced and the shift from the chromaticity point (A) of the A light source is remarkably smaller than that of the (E) point.
The reference (G') designate the chromaticity point as the body color of the phosphor screen of the three color phosphors (2) containing the ZnS:Cu, Au, Al phosphor having larger content of Au as the activator. The shift from the chromaticity point (A) of the A light source is less than that of the (F') point.
In view of the contribution for the stability of the body color of the phosphor screen used with the face plate glass made of the Nd-containing glass. The content of Au as the activator in the ZnS:Cu, Au, Al phosphor is preferably 5×10-5 g. or more to 1 g. of zinc sulfide as the main component. The stability of the body color of the phosphor screen is further improved by the content of Cu of 4×10-4 g. or more to 1 g. of zinc sulfide.
FIGS. 10, 17, 18 show the spectral reflectance curves of the ZnS:Ag blue phosphor with a blue colorant, as a blue (B)
phosphor the Y2 O2 S:Eu phosphor with a red colorant as a red (R) phosphor which are recently used to improve the contrast of the phosphor screen and various green (G) phosphor and the three color phosphors as the combinations (FIGS. 10, 17, 18) and a CIE chromaticity diagram FIG. 19 plotting body colors of the phosphor screens of said three color phosphors formed on the inner surface of the face plate glass made of the clear glass or the Nd-containing glass by using the light of the illuminant A as the white exterior light (FIG. 19) which are shown as FIGS. 6, 14, 15, 16.
In FIGS. 10, 17, 18, the curve (i') is a spectral reflectance curve of the ZnS:Ag phosphor with a blue colorant the curve (j') is a spectral, reflectance curve of the Y2 O2 S:Eu phosphor with a red colorant. A spectral reflectance curve of the three color phosphors (2) as the combination of these blue and red phosphors and the Gd2 O2 S:Tb phosphor is the curve (k') in which the reduction of the light component at 470-480 nm is not substantially found. When the ZnS:Cu, Au, Al phosphor is combined with these blue and red phosphors, the spectral reflectance curve is the curve (1') shown in FIG. 17 in which the light component at 470-480 nm is remarkably reduced.
When the ZnS:Cu, Au, Al phosphor having large content of Au as the activator is combined with these blue and red phosphors, the spectral reflectance curve is the curve (m') shown in FIG. 18 in which the light component at 470-480 nm is further reduced.
FIG. 19 is a CIE chromaticity diagram plotting body colors of the phosphor screens of the three color phosphors as the
combinations thereof which are respectively formed on the face plate glass made of the clear glass or the Nd-containing glass by the light of the illuminant A as the white exterior light. The reference (A) designates the chromaticity point of the illuminant A. The reference (H) designates the chromaticity point of the reflected exterior light from the phosphor screen of the three color phosphors (2) as the combination of the Gd2 O2 S:Tb phosphor, the ZnS:Ag phosphor with a blue colorant and the Y2 O2 S:Eu phosphor with a red colorant formed on the inner surface of the face plate glass (1) made of the clear glass as the chromaticity point of the body color of the phosphor screen. The reference (I) designates the chromaticity point of the reflected exterior light from the phosphor screen of the three color phosphors (2) as the combination of the Gd2 O2 S:Tb phosphor, the ZnS:Ag phosphor with a blue colorant and the Y2 O2 S:Eu phosphor with a red colorant formed on the inner surface of the face plate glass (1) made of the Nd-containing glass as the chromaticity point of the body color of the phosphor screen. The reference (J') designates the chromaticity point of the reflected exterior light from the phosphor screen of the three color phosphors (2) as the combination of the ZnS:Cu, Au, Al phosphor, the ZnS:Ag phosphor with a blue colorant and the Y2 O2 S:Eu phosphor with a red colorant formed on the inner surface of the face plate glass (1) made of the Nd-containing glass as the chromaticity point of the body color of the phosphor screen. The reference (K') designates the chromaticity point of the reflected exterior light from the phosphor screen of the three color phosphors (2) as the combination of the ZnS:Cu, Au. Al phosphor having larger content of Au as the activator, the ZnS:Ag phosphor with a blue colorant and the Y2 O2 S:Eu phosphor with a red colorant formed on the inner surface of the face plate glass (1) made of the Nd-containing glass as the chromaticity point of the body color of the phosphor screen. As it is clearly found from the chromaticity points, the light component at 470-480 nm of the reflected exterior light can be reduced by the combination of the ZnS:Cu, Au, Al phosphor as the green (G) phosphor with the blue colorant-coated blue (B) phosphor and the red colorant-coated red (R) phosphor, whereby the unstable body color caused by the use of the face plate glass (1) made of the Nd-containing glass is remarkably improved. That is, the chromaticity point of the body color is shifted from (I) to (J'). When the content of Au as the activator in the ZnS:Cu, Au, Al phosphor is increased, the stability of the body color of the phosphor screen is further improved by further reducing the light component at 470-480 nm. That is, the chromaticity point is shifted to (K').
The embodiment using the A light source as the white exterior light source has been described. Thus, the same effect is given by reducing the light component at 470-480 nm of the reflected exterior light by the combination of the ZnS:Cu, Au, Al phosphor as the green (G) phosphor in the case of the other white exterior light source such as the white fluorescent lamp and illuminant C.
Recently, a black matrix type phosphor screen having light absorption layer between the three color phosphors has been used for improving the contrast of the phosphor screen. The present invention can be applied for such phosphor screen.
In accordance with the present invention, the unstable body color of the phosphor screen caused by the use of the face plate glass made of the Nd-containing glass can be overcome by the combination of the three color phosphors using the ZnS:Cu, Au, Al phosphor as the green (G) phosphor whereby the phosphor screen for the stable body color is given and the contrast and brightness characteristics are improved to provide a cathode ray tube having high quality.
The face plate glass can be a combination of a main face plate glass made of a clear glass and a front plate glass made of the Nd-containing glass.
Foreign References:
DE2804155A1 1978-08-03 313/467
DE3010386A1 1980-09-25 313/480
DE2807085A1 1979-09-20


















No comments:
Post a Comment
The most important thing to remember about the Comment Rules is this:
The determination of whether any comment is in compliance is at the sole discretion of this blog’s owner.
Comments on this blog may be blocked or deleted at any time.
Fair people are getting fair reply. Spam and useless crap and filthy comments / scrapers / observations goes all directly to My Private HELL without even appearing in public !!!
The fact that a comment is permitted in no way constitutes an endorsement of any view expressed, fact alleged, or link provided in that comment by the administrator of this site.
This means that there may be a delay between the submission and the eventual appearance of your comment.
Requiring blog comments to obey well-defined rules does not infringe on the free speech of commenters.
Resisting the tide of post-modernity may be difficult, but I will attempt it anyway.
Your choice.........Live or DIE.
That indeed is where your liberty lies.
Note: Only a member of this blog may post a comment.